Absolute humidity

If all the water vapor in one cubic meter of air were condensed into a container, the mass of the water in the container could be measured with a scale to determine absolute humidity. The amount of water vapor in that cube of air is the absolute humidity of that cubic meter of air. More technically, absolute humidity on a volume basis is the mass of dissolved water vapor, mw, per cubic meter of total moist air, Vnet:
Absolute humidity ranges from 0 grams per cubic meter in dry air to 30 grams per cubic meter (0.03 ounce per cubic foot) when the vapor is saturated at 30 °C.
The absolute humidity changes as air pressure changes. This is very inconvenient for chemical engineering calculations, e.g. for clothes dryers, where temperature can vary considerably. As a result, absolute humidity is generally defined in chemical engineering as mass of water vapor per unit mass of dry air, also known as the mass mixing ratio (see below), which is much more rigorous for heat and mass balance calculations. Mass of water per unit volume as in the equation above would then be defined as volumetric humidity. Because of the potential confusion, British Standard BS 1339 (revised 2002) suggests avoiding the term "absolute humidity". Units should always be carefully checked. Most humidity charts are given in g/kg or kg/kg, but any mass units may be used.
The field concerned with the study of physical and thermodynamic properties of gas-vapor mixtures is named Psychrometrics.
Relative humidity
Relative humidity is defined as the ratio of the partial pressure of water vapor (in a gaseous mixture of air and water vapor) to the saturated vapor pressure of water at a given temperature. In other words, relative humidity is the amount of water vapor in the air at a specific temperature compared to the maximum water vapor that the air is able to hold without it condensing, at that given temperature. Relative humidity is expressed as a percentage and is calculated in the following manner:

where
 is the partial pressure of water vapor in the gas mixture;
is the partial pressure of water vapor in the gas mixture; is the saturation vapor pressure of water at the temperature of the gas mixture; and
is the saturation vapor pressure of water at the temperature of the gas mixture; and is the relative humidity of the gas mixture being considered.
is the relative humidity of the gas mixture being considered.
Relative humidity is an important metric used in weather forecasts and reports, as it is an indicator of the likelihood of precipitation, dew, or fog. In hot summer weather, a rise in relative humidity also increases the apparent temperature to humans (and other animals) by hindering the evaporation of perspiration from the skin as the relative humidity rises. For example, according to the Heat Index, a relative humidity of 75% at 80°F (27°C) would feel like 83.574°F ±1.3 °F (28.652°C ±1.7 °C) at ~44% relative humidity.
Specific humidity
Specific humidity is the ratio of water vapor to dry air in a particular mass, and is sometimes referred to as humidity ratio. Specific humidity ratio is expressed as a ratio of grams of water vapor, mv, per kilogram of dry air ma .
That ratio is defined as:
Specific humidity can be expressed in other ways including:
or:
Using the definition of specific humidity, the relative humidity can be expressed as
However, specific humidity is also defined as the ratio of water vapor to the total mass of the system in meteorology. "Mixing ratio" is used to name the definition in this section beginning.
Specific enthalpy
Analogous to the specific enthalpy of a pure substance. In psychrometrics, the term quantifies the total energy of both the dry air and water vapor per pound of dry air.
[edit]Specific volume
Analogous to the specific volume of a pure substance. In psychrometrics, the term quantifies the total volume of both the dry air and water vapor per pound of dry air.
[edit]Psychrometric ratio
The psychrometric ratio is the ratio of the heat transfer coefficient to the product of mass transfer coefficient and humid heat at a wetted surface. It may be evaluated with the following equation:[4][5]
- where:
- r = Psychrometric ratio, dimensionless
- hc = convective heat transfer coefficient, W m-2 K-1
- ky = convective mass transfer coefficient, kg m-2 s-1
- cs = humid heat, J kg-1 K-1
The psychrometric ratio is an important property in the area of psychrometrics, as it relates the absolute humidity and saturation humidity to the difference between the dry bulb temperature and the adiabatic saturation temperature.
Mixtures of air and water vapor are the most common systems encountered in psychrometry. The psychrometric ratio of air-water vapor mixtures is approximately unity, which implies that the difference between the adiabatic saturation temperature and wet bulb temperature of air-water vapor mixtures is small. This property of air-water vapor systems simplifies drying and cooling calculations often performed using psychrometic relationships.
Dew Point
A measure of atmospheric moisture. It is the temperature to which air must be cooled in order to reach saturation (assuming air pressure and moisture content are constant). A higher dew point indicates more moisture present in the air. It is sometimes referred to as Dew Point Temperature, and sometimes written as one word (Dewpoint).
The most common type of hygrometer is the dry- and wet-bulb psychrometer . It consists of two identical mercury or electrical thermometers , one of which has a wet cotton or linen wick around its bulb. Evaporating water from the wick absorbs heat from the thermometer bulb, causing the thermometer reading to drop. The difference between dry-bulb and wet-bulb temperatures are compared on psychrometric charts.






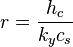

Comments
Post a Comment